Table of Contents
- Introduction
- Fueling Beginnings: The Metabolic Orchestra of Fetal Life
- First Breath, First Fuel: Surviving the Birth-Suckling Shift
- Milk, Mitochondria, and the Making of an Infant Metabolism
- Take-Home Message
- Summary and Conclusion
- Did You Know About Folate Receptor Autoantibodies (FRAAs) and Brain Development?
- References

Figure 1. Metabolism at the Dawn of Life: From Placenta to Independent Physiology. Metabolic Transitions at Birth (Fetal Metabolism → Birth-Suckling Transition → Neonatal Metabolism). (1) Fetal Metabolism: Fetal growth depends on placental glucose transfer, orchestrated by hormones like insulin and IGFs; limited oxygen favors oxidative glucose use over fatty acid oxidation. These early metabolic patterns influence lifelong health outcomes. (2) Birth–Suckling Transition: The abrupt loss of placental support triggers glycogenolysis and rapid gluconeogenesis, powered by hormonal shifts and nutrient mobilization — a metabolic pivot essential for newborn survival. (3) Neonatal Metabolism: Postnatal adaptation centers on milk-derived energy, with fatty acid β-oxidation and ketone utilization dominating; brown adipose tissue fuels thermogenesis, setting the stage for independent energy homeostasis.
Introduction
Before breath, before warmth, and before first suckle, human life is orchestrated by an invisible metabolic symphony. From the earliest fetal stirrings to the neonatal gasp of independence, energy flow governs every step of development. Metabolism in early life is not merely a background process—it is the foundation of growth, survival, and long-term health.
In the womb, the fetus exists in a state of remarkable biological interdependence, fully reliant on maternal nutrients delivered via the placenta—a high-demand organ with its own energy needs. The placenta not only transfers essential carbohydrates, amino acids, and lipids, but also regulates waste removal and balances oxygenation in a low-oxygen environment. As gestation unfolds, fetal tissues adapt their nutrient preferences: glucose, with its oxygen efficiency, dominates; glycogen reserves expand in the liver; and metabolic enzymes prepare quietly for life after birth (see Figure 1; Figure 2) [1].
But with birth comes a seismic shift. Nutritional supply from the placenta halts instantly, launching the neonate into a precarious transition where survival depends on internal stores and the rapid onset of lactation. Brown adipose tissue ignites, the liver begins gluconeogenesis, and previously quiet metabolic pathways surge to restore energy balance. Hormonal landscapes shift—insulin drops, glucagon rises, and the newborn rewires its energy machinery, step by step.
As neonatal metabolism matures—eventually resembling adult patterns—research continues to reveal the profound impact of these early metabolic decisions on lifelong health, from insulin sensitivity to neurodevelopment. This article traces the intricate choreography of fetal and neonatal metabolism, revealing the critical roles of tissue fuel choice, hormonal timing, and biochemical adaptation in shaping the beginnings of human life.
I. Fueling Beginnings: The Metabolic Orchestra of Fetal Life
Before birth, a human fetus relies entirely on a lifeline of nutrients and oxygen supplied by the placenta, a dynamic interface between mother and child. This organ not only nourishes, but also manages waste removal from the growing fetus—and remarkably, its own metabolic hunger can rival the fetus’s, occasionally consuming fetal resources when maternal supplies run low. Despite challenges in studying human fetal metabolism directly, experimental models offer robust insights.
The Placenta: Mother’s Metabolic Ambassador
The placenta, boasting a sprawling surface area of about 11 m² at birth, governs fetal access to carbohydrates, amino acids, lipids, and micronutrients. These nutrients cross the placental barrier via:
- Simple diffusion (e.g., for oxygen and water)
- Transporter-mediated uptake (notably for glucose)
- Pinocytosis (for macromolecules like proteins)
As a conduit of life, the placenta ensures that nutrient supply aligns with maternal cardiovascular health and metabolism, highlighting the deep interdependence of mother and fetus.
Metabolic Priorities: Growth Over Movement
Though tiny, the fetus is an energetic powerhouse. Energy is devoted mostly to tissue growth and cellular metabolism, with relatively little spent on movement or heat regulation. Surprisingly, fetal basal metabolic rate—measured via placental O₂ and CO₂ exchange—remains stable across a wide spectrum of maternal glucose and oxygen levels. This constancy, however, varies among species. Humans, known for their slow fetal development, favor oxidative metabolism, with respiratory quotients near 1, indicating a strong reliance on glucose as the primary fuel (see Figure 2).
Why Glucose Reigns: Oxygen Efficiency Matters
In the womb’s low-oxygen (hypoxic) environment, glucose shines as a more oxygen-efficient fuel than fatty acids. This explains why fetal heart tissue, which later thrives on fatty acids, depends almost exclusively on glucose during gestation. The high glucose requirement (about 4–8 mg/kg/min) is met via GLUT1 and GLUT3 transporters, maintaining a fetal-to-maternal concentration ratio of roughly 70–80% [2-4].
Supporting this uptake is fetal insulin, secreted by the developing pancreas, which enhances glucose utilization. Alongside insulin-like growth factors (IGFs), which respond to nutrient availability, these hormones orchestrate the tempo of fetal growth. The fetal capacity to adjust to nutrient shifts may lay the groundwork for adult metabolic disorders—a field of growing scientific interest.
Lactate and Glycogen: Strategic Reserves Before Birth
Contrary to earlier views, the fetus does not just produce lactate anaerobically; it likely utilizes lactate supplied by the placenta, covering nearly one-third of its energy needs. Meanwhile, the fetal liver begins synthesizing and hoarding glycogen late in gestation. Glycogen concentrations can reach 180 mg/gram of liver, far exceeding adult levels and rivaling those seen in glycogen storage diseases. This surge prepares the newborn for suckling and energy demands post-birth.
Interestingly, glycogen breakdown enzymes remain mostly dormant until birth, except under maternal metabolic stress, such as hypoglycemia, when the fetus may tap into its own reserves—even to aid the placenta. While gluconeogenic enzymes are present in late-stage fetuses, their activity remains minimal until birth.
Protein Builders: Amino Acids in Fetal Nutrition
Amino acids are essential not only for constructing proteins but also as secondary energy sources, compensating for the limits of glucose metabolism (see Figure 2). Fetal urea production hints at this dual role. These amino acids cross the placenta, yet the transfer is not a simple reflection of maternal supply. In sheep models [5-7], for instance:
- Neutral/basic amino acids move into fetal circulation
- Acidic amino acids (e.g., glutamate) flow from fetus to placenta
Though such patterns likely apply to humans, direct evidence remains elusive. Moreover, the amniotic fluid serves as a bonus nutrient reservoir, offering amino acids absorbed through fetal skin and swallowed during development.
Lipids and Limits: Fats Without Fire
Although fatty acid oxidation is subdued in utero due to hypoxia, fatty acids—especially essential ones—are critical for growth. These are imported via:
- Dedicated transporters
- “Flip-flop” diffusion mechanisms
Notably, triacylglycerols rarely cross the placenta intact (see Figure 2). Instead, the placenta breaks down maternal lipoproteins, extracting fatty acids and cholesterol, which shape the fetal lipid profile—uniquely human compared to other mammals. In the final gestational stages, the fetal liver begins lipid synthesis, and brown adipose tissue expands in readiness for postnatal thermoregulation.
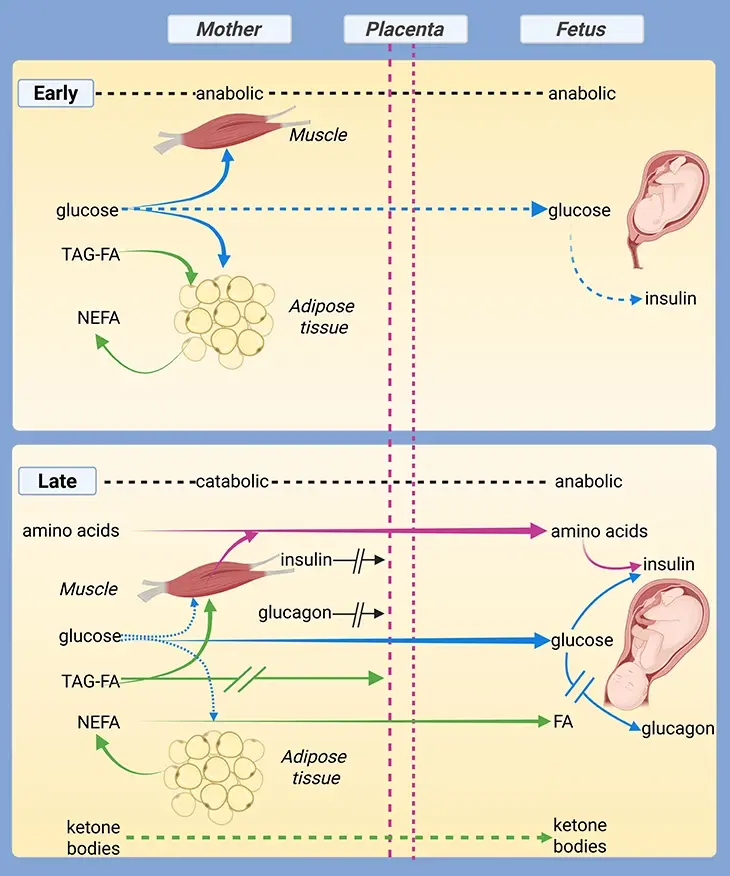
Figure 2. Nutrient Utilization in the Developing Fetus. While all three macronutrient classes—carbohydrates, proteins, and fats—contribute to fetal growth, glucose is presumed to be the predominant energy source. Arrow thickness represents relative flux, indicating the magnitude of nutrient transfer and metabolic activity. Nourishing Beginnings: as soon as a baby begins to form, a quiet miracle unfolds. Every heartbeat of the mother, every bite she takes, carries life-giving nutrients that cross through the placenta—a bridge between her world and her baby’s. Of all the nutrients, glucose acts like a gentle spark, energizing the rapid growth of tiny limbs, organs, and the forming brain. Proteins build strength, fats shape resilience, but glucose is the baby’s main fuel, flowing steadily to meet the rising energy needs. In this figure, you will see how your baby uses these nutrients. The thicker arrows highlight the pathways that work hardest—showing how your body lovingly prioritizes what your baby needs most.
II. First Breath, First Fuel: Surviving the Birth-Suckling Shift
The moment a neonate leaves the protective confines of the womb, a profound metabolic shift is underway. Gone is the placental pipeline of nutrients and oxygen—and in its place, a brief but precarious gap before lactation begins. This window, often spanning a few critical hours, demands that the newborn draw upon its internal fuel reserves to survive.
The Glycogen Lifeline: Energy in the Liver
As a biological safety net, the fetus stockpiles substantial quantities of hepatic glycogen during late gestation. This glucose polymer serves as the primary emergency fuel during the abrupt transition from womb to world. Once born, the neonate experiences a dramatic spike in glucose utilization, estimated around 4–8 mg/kg/min, roughly twice that of an adult.
Consequently, blood glucose levels plummet rapidly, dropping to nearly 2 mmol/L within the first two hours of life. This precipitous decline triggers:
- 📉 Decreased insulin levels
- 📈 Rising glucagon and catecholamine concentrations
The resulting low insulin-to-glucagon ratio catalyzes hepatic glycogenolysis—breaking down glycogen into glucose—which restores plasma glucose to around 4–5 mmol/L by six hours of age.
However, this glycogen reserve is finite. If feeding is not established within 12 hours, the liver’s supply becomes exhausted, risking fatal hypoglycemia, as neonates have limited tolerance for fasting compared to adults.
Activating Plan B: The Rise of Gluconeogenesis
Thankfully, a secondary pathway—gluconeogenesis—ramps up rapidly in the hours after birth. Initially accounting for about 10% of glucose turnover, it gains momentum as gluconeogenic enzymes are upregulated within the first 24 hours post-partum.
The primary substrates fueling this process include:
- Amino acids, especially alanine
- Glycerol from modest lipolysis
- Lactate, contributing a minor share
This metabolic agility reflects the newborn’s need to bridge nutritional gaps until suckling is reliably established. It also highlights the remarkable plasticity of neonatal metabolism—ready to adapt to dramatic shifts in energy supply within mere hours.
III. Milk, Mitochondria, and the Making of an Infant Metabolism
Once the umbilical lifeline is severed and lactation begins, the neonate embarks on its first solo metabolic journey. Milk, rich in fat and tailored by evolution, becomes the sole source of energy—a biochemical bridge from fetal dependence to independent living. This shift is not just about new nutrients. It rewrites the hormonal script and fundamentally reconfigures how the neonate generates and allocates energy.
From Fetal Quietude to Neonatal Anabolism
With suckling underway, nutrient absorption tips the insulin–glucagon ratio back toward anabolism, reigniting pathways for growth and tissue building. This hormonal rebalancing activates cellular processes once dimmed during the stress of birth—signaling a return to constructive metabolism.
But beyond hormonal changes, the neonate now faces responsibilities previously managed by the mother or placenta: the most immediate being thermoregulation.
Firing Up Brown Fat: The Neonate’s Inner Heater
Brown adipose tissue (BAT)—known for its rich mitochondrial content and capacity for non-shivering thermogenesis—becomes metabolically active at birth. This tissue was largely dormant in utero, where external heat from the maternal body sufficed. Now exposed to cooler surroundings, the neonate must generate heat independently, using uncoupled oxidative phosphorylation in brown fat to maintain body temperature.
The oxygen-dependent nature of this process dovetails with a key physiological transformation: the closure of fetal circulatory shunts—foramen ovale and ductus arteriosus—which increase oxygen delivery to peripheral tissues [8]. As the neonate starts breathing, oxygen availability surges, allowing a shift toward more oxidative energy generation.
Fat Burns Bright: The Rise of β-Oxidation
With enhanced oxygenation, fatty acid β-oxidation ascends as the dominant pathway for ATP production in tissues like the muscle and kidney. Even the heart, which in fetal life exhibited hypoxia resistance, gradually becomes more reliant on fatty acid oxidation—a metabolic signature of adult myocardium.
Studies indicate the neonate carries about 16% of its birth weight as adipose tissue, a reserve critical for both energy and heat. Interestingly, infants born to diabetic mothers often display excess fat stores, potentially due to hyperglycemia-induced lipogenesis and elevated fatty acid exposure in utero [9-12].
Brain Fuel Reimagined: Ketones Take Center Stage
In a notable twist, the neonatal brain exhibits a greater capacity to utilize ketone bodies than its adult counterpart. These molecules—byproducts of fatty acid oxidation—can supply 10–15% of cerebral energy in the suckling-fed state. Researchers believe this is an evolutionary adaptation to the high-fat composition of milk, which induces a mild, physiological ketosis in neonates.
Ketones not only meet energetic demands but serve as biosynthetic precursors for brain development, contributing to lipid membranes and myelination—key to neural maturation.
Approaching Equilibrium: Metabolic Maturity by Weaning
As the infant transitions from milk to solid foods at weaning, its metabolic profile steadily aligns with that of an adult. Glucose becomes the primary fuel, hormonal balance stabilizes, and reliance on ketones and brown fat diminishes. By this stage, the metabolic system has morphed from a finely tuned fetal machine into a flexible, self-sustaining engine—ready to adapt to life outside the womb.
Take-Home Message
Fetal Metabolism
- Placental nutrient transfer is central to fetal growth, with glucose serving as the dominant fuel in a low-oxygen uterine environment.
- The fetus accumulates hepatic glycogen in late gestation to buffer against energy demands during birth.
- Fetal energy metabolism is governed by oxidative glucose utilization, supplemented by lactate and amino acids, while fatty acid oxidation remains low.
- Hormonal cues such as insulin and IGFs regulate fetal growth in response to nutrient availability.
Birth–Suckling Transition
- Placental supply halts abruptly, triggering a metabolic emergency where hepatic glycogen is mobilized to prevent hypoglycemia.
- Glucose utilization rate peaks, while falling insulin and rising glucagon activate glycogenolysis and initiate gluconeogenesis.
- Gluconeogenic activity surges postnatally, supported by amino acids, glycerol, and lactate.
- If feeding is delayed, rapid glycogen depletion can result in life-threatening hypoglycemia.
Neonatal Metabolism
- With milk established as the sole energy source, nutrient absorption shifts the hormonal balance back to anabolism and growth.
- Increased oxygen availability allows fatty acid β-oxidation to become the dominant energy pathway in muscle, kidney, and heart.
- Brown adipose tissue activates for non-shivering thermogenesis, essential for postnatal temperature regulation.
- The neonatal brain efficiently utilizes ketone bodies, reflecting adaptation to the high-fat composition of milk.
- By weaning, the infant’s metabolism resembles adult energy dynamics, completing the transition to independent metabolic control.
(Cf. previous blogs entitled as: “Developmental Origins of Health and Disease: Microbiomes, Neurodevelopment, and Behavior.”; “The Brain on Food: Rethinking Mental Health from the Inside Out.”)
Summary and Conclusion
The metabolic transition from fetal reliance to neonatal independence is one of the most critical and complex physiological events in human life. Governed by the cessation of placental support and the abrupt demand for self-sustained energy production, this shift is mediated through tightly regulated hormonal cascades, tissue remodeling, and enzyme activation. Placental metabolism, previously a silent orchestrator, gives way to neonatal processes such as hepatic gluconeogenesis, lipolysis, and thermogenesis, with brown adipose tissue and the neonatal liver assuming central roles in survival.
Notably, recent research has expanded our understanding of mitochondrial biogenesis and function during perinatal transition, highlighting its indispensable role in sustaining ATP production amidst oxidative challenges. Studies also point to epigenetic reprogramming during this period, with long-term implications for insulin sensitivity, adipocyte function, and susceptibility to metabolic disorders. The interplay between nutritional status, hormonal signaling, and gene expression in this window is increasingly recognized as a determinant of health trajectories well into adulthood.
Despite these advances, significant gaps remain. The precise molecular drivers of tissue-specific metabolic reprogramming, especially in vulnerable populations such as preterm infants, remain poorly defined. Likewise, the impact of maternal metabolic conditions (e.g., diabetes, obesity) on fetal programming and neonatal adaptation demands deeper mechanistic exploration. Emerging technologies — from single-cell metabolomics to placental organoids — offer promising avenues to decode these processes at unprecedented resolution.
Future efforts should prioritize integrative models that connect developmental metabolism, mitochondrial dynamics, and cellular energetics with long-term outcomes in neurodevelopment and disease resilience. This framework could not only inform neonatal care practices but also shape therapeutic interventions aimed at optimizing early-life metabolic trajectories.
In essence, the metabolic switch at birth is not merely a survival mechanism — it is the template upon which health is inscribed.
For information on autism monitoring, screening and testing please read our blog.
References
- Rao PN, Shashidhar A, Ashok C. In utero fuel homeostasis: Lessons for a clinician. Indian J Endocrinol Metab. 2013 Jan;17(1):60-8. doi: 10.4103/2230-8210.107851. PMID: 23776854; PMCID: PMC3659908.
https://pubmed.ncbi.nlm.nih.gov/23776854/ - Shelley HJ. Carbohydrate metabolism in the foetus and the newly born. Proc Nutr Soc. 1969 Mar;28(1):42-9. doi: 10.1079/pns19690008. PMID: 4891848.
https://pubmed.ncbi.nlm.nih.gov/4891848/ - Şengül Ö, Dede S. Maternal and Fetal Carbohydrate, Lipid and Protein Metabolisms. Eur J Gen Med 2014; 11(4):299-304. DOI : 10.15197/sabad.1.11.93.
https://www.ejgm.co.uk/download/maternal-and-fetal-carbohydrate-lipid-and-protein-metabolisms-7180.pdf - Bowman CE, Arany Z, Wolfgang MJ. Regulation of maternal-fetal metabolic communication. Cell Mol Life Sci. 2021 Feb;78(4):1455-1486. doi: 10.1007/s00018-020-03674-w. Epub 2020 Oct 21. PMID: 33084944; PMCID: PMC7904600.
https://pubmed.ncbi.nlm.nih.gov/33084944/ - Thureen PJ, Hay WW, Thureen PJ. Preface. In: Hay WW, ed. Neonatal Nutrition and Metabolism. Cambridge University Press; 2006:xvii-xviii.
https://api.pageplace.de/preview/DT0400.9780511189920_A23689969/preview-9780511189920_A23689969.pdf
- Bloomfield FH, Jaquiery AL, Oliver MH. Nutritional regulation of fetal growth. Nestle Nutr Inst Workshop Ser. 2013;74:79-89. doi: 10.1159/000348405. Epub 2013 Jul 18. PMID: 23887106.
https://pubmed.ncbi.nlm.nih.gov/23887106/ - Wallace JM, Bourke DA, Aitken RP, Milne JS, Hay WW Jr. Placental glucose transport in growth-restricted pregnancies induced by overnourishing adolescent sheep. J Physiol. 2003 Feb 15;547(Pt 1):85-94. doi: 10.1113/jphysiol.2002.023333. Epub 2002 Aug 23. PMID: 12562948; PMCID: PMC2342623.
https://pubmed.ncbi.nlm.nih.gov/12562948/
- Scholz TD, Segar JL. Cardiac Metabolism in the Fetus and Newborn. Neoreviews (2008) 9 (3): e109–e118.
https://doi.org/10.1542/neo.9-3-e109
- Niemi AK. Neonatal Presentations of Metabolic Disorders. Neoreviews. 2020 Oct;21(10):e649-e662. doi: 10.1542/neo.21-10-e649. PMID: 33004558.
https://pubmed.ncbi.nlm.nih.gov/33004558/
- Desai M, Hales CN. Role of fetal and infant growth in programming metabolism in later life. Biol Rev Camb Philos Soc. 1997 May;72(2):329-48. doi: 10.1017/s0006323196005026. PMID: 9155245.
https://pubmed.ncbi.nlm.nih.gov/9155245/
- Asakura H. Fetal and neonatal thermoregulation. J Nippon Med Sch. 2004 Dec;71(6):360-70. doi: 10.1272/jnms.71.360. PMID: 15673956.
https://pubmed.ncbi.nlm.nih.gov/15673956/ - Ringer, SA. Core Concepts: Thermoregulation in the Newborn Part I: Basic Mechanisms. Neoreviews (2013) 14 (4): e161–e167.
https://doi.org/10.1542/neo.14-4-e161